Abstract
This application note describes the use of a KP5000 Kelvin probe (McAllister Technical Services) with associated electronics and software to measure the work function change of a surface as the temperature is increased to induce surface reactions. While the reactants and products of the surface reaction must be established by independent methods, the high sensitivity of the surface work function to the composition and structure of adsorbates on surface makes this approach an extremely effective method for monitoring the reactant to product transformation on the surface in real time. The high precision of the KP5000 probe (standard deviation of repeat measurements on the same surface monolayer of <3 meV) and the phase-locking control of the associated electronics and software make measurements such as those illustrated below routine.
Introduction
One of the challenges in studying surface reactions is to measure the transformation from reactants to products in real time. In cases where one or more of the reaction products desorb from the surface, mass spectrometric detection has proven to be a straightforward and useful approach for monitoring the reaction. In cases where none of the products desorb from the surface, however, detecting the reaction and monitoring it in real time is much more difficult. Spectroscopic signatures for the reactants and products are often hard to distinguish or deconvolve, and doing so in real time is particularly challenging.A simple and relatively inexpensive way to monitor the reactant to product transformation is through measurement of the change of the surface work function using a Kelvin probe [1]. Changes in the coverage, bonding, and structure of adsorbates on surfaces are accompanied by changes in the surface work function, and even subtle changes such as phase transitions in the adsorbed layer are often accompanied by a work function change of 30 meV or more. With a Kelvin probe, changes in the surface work function of less than 30 meV are readily detectable, and a precision of a few meV is possible. Furthermore, with state-of-the-art electronics and software, these measurements can be made in seconds or less, so that real-time monitoring of surface reactions is possible.
This application note describes the use of a Kelvin probe for real-time monitoring of surface reactions when the surface is heated at a rate of 0.1 K/s. The reactions that will be illustrated were carried out on a Cu(100) surface and are carbon-bromine bond dissociation in vinyl bromine followed by vinyl coupling to produce butadiene (equation 1) and carbon-bromide bond dissociation in cyclopentyl bromide followed by ß -hydride elimination and subsequent cyclopentene desorption (equation 2).
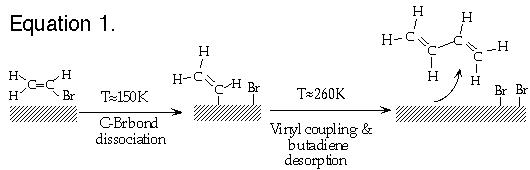
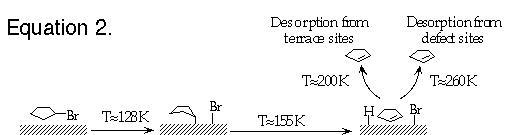
Both reaction pathways involve a series of sequential steps which produce subtle changes in surface spectroscopic signatures. Extensive studies (some illustrated below) have thus been carried out to verify the reaction steps and reaction temperatures which are shown above. In the results presented here, we show how these reaction temperatures can be much more simply determined by using a KP5000 Kelvin probe to measure the work function change as the surface is heated to induce the reactions.
Experimental
The work function change measurements were made using a KP5000 Kelvin probe (McAllister Technical Services). This apparatus measures the contact potential difference (CPD) between a reference plate and the surface of interest. The CPD is defined as the potential difference between the surfaces of the reference plate and the sample (Vref - V samp, where V is the surface potential). The work function change Δwf with respect to the clean surface is then obtained from:
Δwf = wfad - wfclean = CPDclean - CPDads = (Vref - Vclean) - (Vref - Vad)where (Vref - Vclean)where (Vref - Vclean) is the contact potential difference of the clean surfaces and (Vref - Vad) is the contact potential difference of the adsorbate-covered surface.
To measure the surface work function change, the KP5000 probe is electrically connected via ground to the sample. The reference plate of the probe is positioned ~1 mm from the sample and driven (in the studies here) at an oscillation frequency of 90 Hz. An alternating current flows in the external circuit when there is a non-zero electric field (a contact potential difference) between the sample and the reference plate. The KP 5000 electronics use phase locking detection and signal averaging to determine the AC current amplitude as a function of the backing potential applied to the reference plate. In the studies reported here, 10 measurements were averaged for each applied potential. Since the amplitude of the AC current changes linearly with the backing potential and is zero when the backing potential matches the contact potential difference between the reference plate and the sample, one can determine the CPD by measuring the current amplitude as a function of the backing potential. The KP 5000 software uses a linear fit to the data to determine the zero amplitude point. In the studies here, the backing potential was stepped in increments of 200 mV over a range of 4 V. The net data acquisition rate (sum of 10 current amplitude measurements at each of 21 backing potentials) was ~14 sec/CPD measurement.
The thermal desorption and work function studies were carried out in an ultra-high vacuum system[2] using a Cu(100) crystal. The crystal was mounted on a resistive heating element by fastening a chromel wire (threaded in a groove in the edge of the crystal) around the heating element. The surface temperature was measured by a chromel-alumel thermocouple junction wedged into a hole drilled in the side of the crystal. The crystal could be cooled through contact with a liquid nitrogen-cooled reservoir to ~110 K.
In the temperature-programmed work function change measurements, a temperature programmer (Eurotherm 818P) and DC power supply were used to achieve a linear heating rate of 0.1K/s. Since the Kelvin probe measures the contact potential difference between the surface and the reference plate, it is important to make sure that the crystal either stays grounded during heating or that the applied potential changes in a small and reproducible way. In the studies here, the sample was grounded through the heater leads outside the chamber so the crystal potential changed slightly with heating. Because the KP 5000 probe uses modulated detection and because the heating current was DC, this offset, as shown by the clean surface curve in Figure 1, was readily determined.
Results
1. Vinyl Bromide (CH2=CHBr) Reaction on Cu(100)
Before presenting the work function change measurements for vinyl bromide on Cu(100), the surface reactions of this molecule are briefly reviewed. Near-edge x-ray absorption fine structure (NEXAFS) measurements at the National Synchrotron Light Source of Brookhaven National Laboratory as well as chemical reaction studies at Columbia University have established that the carbon-bromine bond in vinyl bromide dissociates at ~160 K to produce adsorbed vinyl groups (Ch2=CH) and bromine atoms on the surface. Some of the NEXAFS results used in reaching this conclusion are shown in Figure 1.
The two NEXAFS spectra shown in Figure 1 were taken of the carbon K-edge before and after dissociation of the C-Br bond in vinyl bromide. Both spectra were taken with direction of propagation of the polarized light perpendicular to the surface as shown by the inset schematic. In the spectrum taken at 95 K, the vinyl bromide molecule remains intact on the surface as evidenced by the σ*(C-Br) resonance at 287.8 eV. This conclusion is confirmed by independent studies where the vinyl bromide molecules are displaced from the surface by a more strongly adsorbing molecule such as benzene. The fact that the 287.8 eV transition dominates the normal incidence NEXAFS spectrum at 95 K indicates that the molecules bond to the surface with their molecular plane approximately parallel to the surface plane as shown by the schematic adjacent to the spectrum.
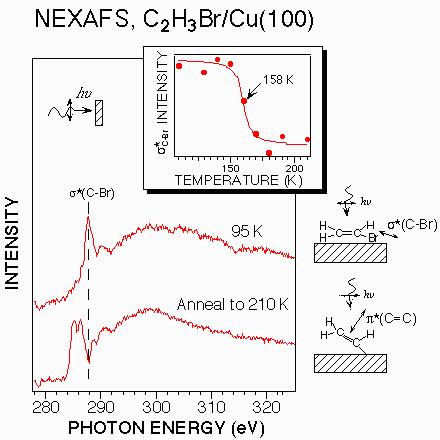
Upon heating the vinyl bromide monolayer in Figure 1 to 210 K, the 278.8 eV σ*(C-Br) resonance is no longer observed in the NEXAFS spectrum (at any angle of incidence). The two peaks observed at 285.0 and 286.4 eV are due to the Π*(C=C) resonances of the double bond in the adsorbed vinyl groups. These resonances are also observed in spectra taken at 95 K when the x-rays are incident on the surface at glancing angles. They are absent in the 95 K spectrum taken at normal incidence because, for the flat-lying geometry of intact vinyl bromide, the transition dipole for the Π*(C=C) transition is orthogonal to the oscillating electric field of the synchrotron radiation. These Π*(C=C) resonances become observable in the normal incidence spectrum at 210 K because the vinyl groups bond with an inclined orientation on the surface as shown in the schematic adjacent to the spectrum.Figure 1: Near edge X-ray adsorption fine structure spectra (normal incidence irradiation) of the carbon K-edge for a partial monolayer of vinyl bromide on Cu(100) at 95 K and 210 K. The schematic diagrams indicate the adsorption geometry and the polarization dependence of the x-ray adsorption transitions which are observed. The inset plot shows the temperature dependence of the σ*(C-Br) resonance intensity; the decrease between 145 and 175 K indicates C-Br bond dissociation.
The inset plot in Figure 1 shows the intensity of the σ*(C-Br) transition in the normal incidence NEXAFS spectra as a function of the temperature to which the surface has been briefly annealed. The intensity drops to zero between 145 and 175 K, indicating dissociation of the C-Br bond over this temperature range. Separate studies show that molecular vinyl bromide can no longer be displaced from the surface by benzene after heating above 175 K, consistent with C-Br bond dissociation. Temperature programmed desorption studies show that the surface vinyl groups generated by C-Br bond dissociation couple above 200 K to form butadiene (CH2=CH-CH=CH2) which is evolved from the surface at ~260 K. This temperature is only slightly higher than that for butadiene desorption from Cu(100), so it is not completely clear from these TPD experiments whether the surface coupling reaction or product desorption is the rate-determining step in butadiene evolution. No carbon is left on the surface after this reaction, but the bromine atoms remain adsorbed until temperatures above 900 K.
The reaction temperatures determined above by an extensive combination of NEXAFS spectroscopy and thermal desorption measurements can be established much more simply by temperature programmed measurements of the surface work function change using a KP5000 Kelvin probe. Figure 2 shows these results. The contact potential difference as a function of surface temperature is shown in Figure 2A by circles. Also shown in Figure 2A is a control experiment on the clean surface. The slope to the clean surface spectrum is attributable to the change in the surface potential because of the DC voltage applied to the sample heater as described in the experimental section. This reproducible effect is accounted for in determining the surface work function change by subtracting the contact potential difference of the adsorbate-covered surface from that of the clean surface (see Experimental Section). The result is shown in Figure 2B.
As shown by the results in Figure 2B, the initial adsorption of 80% of a monolayer of vinyl bromide decreases the surface work function by ~300 meV. This decrease in the surface work function is typical for the adsorption of polar alkyl halides on metal surfaces[3]. The work function increases between 140 and 175 K and between 240 and 280 K are indicative of C-Br bond dissociation and butadiene evolution, respectively. Thus, in a single 30 minute experiment, one obtains virtually all of the temperature-dependent information which required many hours of NEXAFS and TPD studies. Furthermore, since the surface work function is constant to within 10 meV up to the 240 K temperature where butadiene is evolved from the surface, one can conclude with a high level of confidence that the rate butadiene evolution is controlled by the rate of vinyl coupling to form butadiene as opposed to the rate of butadiene desorption -- an issue which could not be resolved from the TPD results alone.
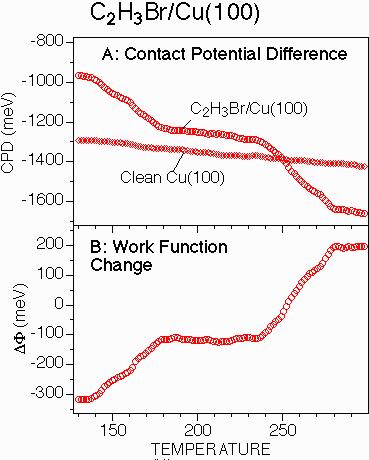
Figure 2: (A) Contact potential difference (CPD) between the Kelvin probe reference plate and a Cu(100) surface as a function of surface temperature before and after adsorption of a partial monolayer of vinyl bromide. (B) Surface work function change obtained from the difference between the two CPD curves in (A). The surface reactions responsible for the changes in work function are given in equation 1.
2. Cyclopentyl Bromide (C5H9Br) reaction on Cu(100)
As with vinyl bromide, the chemistry of cyclopentyl bromide on Cu(100) has been investigated by a variety of techniques to establish the surface reaction steps and to determine the temperatures at which the reactions occur [4]. These results are summarized by the reaction sequence shown previously in equation 2. The reaction temperatures indicated in equation 2 are substantiated by the temperature - programmed surface work function change measurements shown in Figure 3. Also shown in Figure 3 for comparison are thermal desorption results (lower trace) and displacement studies (upper curves) used to corroborate the work function change measurements.
As indicated by the results in Figure 3, adsorption of ~50% of a monolayer of the intact cyclopentyl bromide molecules at ~105 K decreased the surface work function by ~500 meV. Upon heating the surface, the work function increases by ~180 meV between 115 and 145 K as the C-Br bonds dissociate to form adsorbed cyclopentyl groups and bromine atoms on the surface. The work function then decreases by ~80 meV at ~160 K as the adsorbed cyclopentyl groups undergo ß -hydride elimination to produce cyclopentene (C5H8) and H on the surface. Finally, the surface work function increases again above 170 K as the cyclopentene begins to desorb from the surface. The two inflection points in the work function change at ~190 K and at ~240 K correspond to cyclopentene desorption from the (100) terrace sites and defect sites, respectively, as confirmed by independent studies of cyclopentene adsorption on this Cu(100) surface. Again, a single experiment in which the temperature dependence of the surface work function is measured gives all the temperatures where reactions occur.
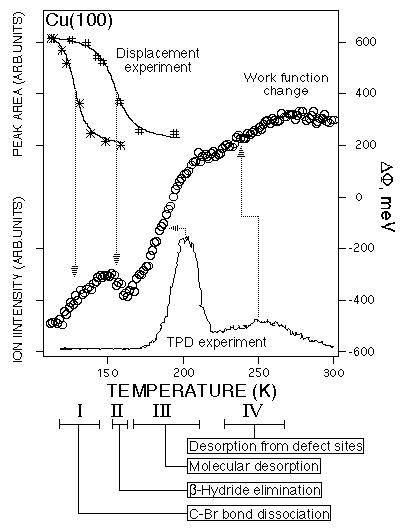
Figure 3: The open circles indicate the change in the surface work function as the temperature of a Cu(100) surface is increased at a rate of 0.1 K/s after adsorption of ~50% of a monolayer of cyclopentyl bromide at 105 K. The arrows indicate the correspondence between the changes in the surface work function and surface reactions as detected by other methods (thermal desorption in the lower trace and chemical displacement experiments in the upper curves). The TPD results are shifted to higher temperatures by 10-15 K due to a faster heating rate of 3 K/s. The surface reaction processes and the temperature ranges over which they occur are indicated beneath the temperature axis as well as in equation 2 of the text.
The results in Figure 3 underscore an important point which must be taken into account when analyzing temperature-dependent work function change measurements to obtain quantitative kinetic parameters for a surface reaction: the surface work function change is often not linearly related to the surface coverage of adsorbates. Nonlinearities can arise from bonding at different sites (as in the case of the cyclopentene desorption in Figure 3) or simply from changes in adsorbate-adsorbate interactions as a function of surface coverage. These types of effects must be quantified in control experiments in order to extract quantitative kinetic information from temperature programmed work function change measurements such as those shown in Figures 2 and 3.
Acknowledgments
We thank the National Science Foundation (grant CHE-93-18635) and the Joint Services Electronics Program administered by the Columbia Radiation Laboratory (contract DAAL 03-91C-0-0016) for financial support.
References
1. J.W. He and P.R. Norton, J. Chem. Phys., 89 (1988) 1170, and references therein.2. J.-L. Lin and B.E. Bent, J. Phys. Chem. 96 (1992) 8529.
3. X.-L. Zhou, F. Solymosi, P.M. Blass, K.C. Cannon and J.M. White, Surf. Sci. 219 (1989) 294.
4. A. Teplyakov and B.E. Bent, manuscript in preparation.