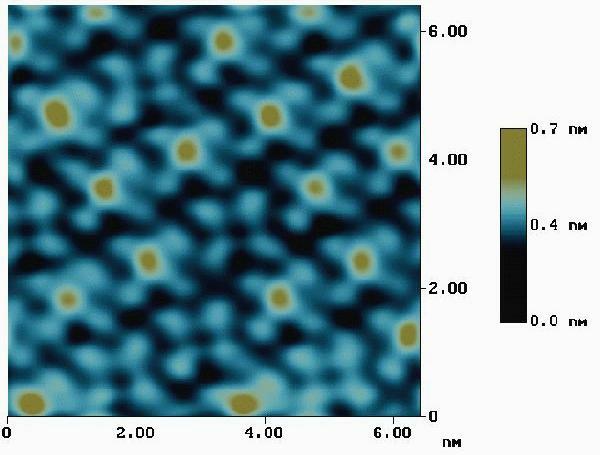
While the acquisition of sub-molecular structure by STM has been a reality for years, the instances where one could clearly determine the chemical nature of a surface species have been few and problematic. In the series of images presented here, one can clearly identify cobalt(II) phthalocyanine [CoPc] from copper(II) phthalocyanine [CuPc]. While these two species are almost identical from a crystallographic perspective, they differ in one very dramatic way - their d-orbital occupations. Cobalt(II) is a d7 system having a half filled dz2 orbital. This orbital can be used to carry charge across the plane of the molecule, and the energy required to fill it is similar to the Fermi energy of the sample. Copper(II), on the other hand, is a d9 system, and addition of an electron to the dx2-y2 orbital requires several eV more energy than the Fermi energy. The effects of this can be seen in the actual STM data taken from a monolayer of CoPc adsorbed on Au(111) [shown below]. Also shown in the figure are the images of three space-filling models of CoPc. Note the very high central regions associated with the partially filled dz2 orbital.
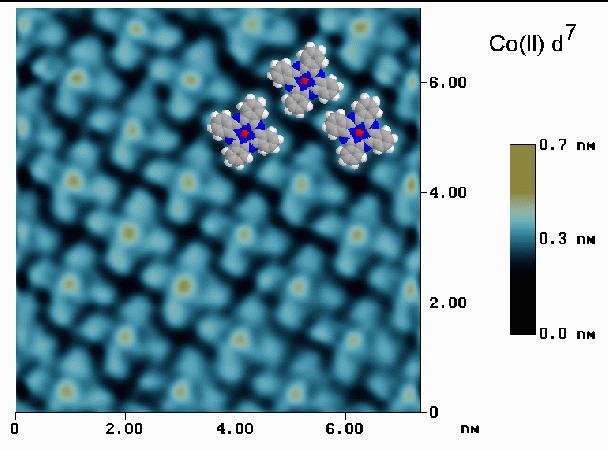
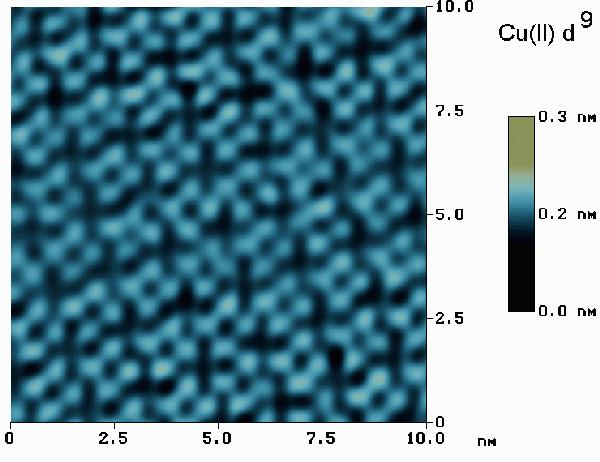
When a monolayer of CuPc on Au(111) is imaged by STM (as shown below), the d orbitals are unable to participate in the tunneling current and the Cu(II) ion appears as a hole in the center of each 4-leaf clover shaped molecule. Since the ionic diameters of Co(II) and Cu(II) differ by less than 0.02 nm, the hight differences are clearly due to the details of d orbital occupation. (See: J. Phys. Chem., June 1996: K.W. Hipps, Xing Lu, X.D. Wang, and Ursula Mazur).
This difference in d-orbital occupation can be used to clearly identify individual molecules adsorbed on surfaces. This is demonstrated in the Figure below wherein a mixed composition layer of CoPc and CuPc was formed on Au(111). It is immediately obvious which are the CoPc (bright centers) and which are the CuPc (dark centers). Moreover, this image difference can be used to monitor surface properties such as diffusion and multi-layer formation (See: J.Amer. Chem. Soc. 1996: K.W. Hipps, Xing Lu, X.D. Wang, and Ursula Mazur).